Recently, Professor Zhang Guoqing's team from the National Research Center for Microscale Matter Science at the University of Science and Technology of China in Hefei reported the discovery of a new mode of interaction between organic molecules: a stable photo induced charge transfer complex can be formed between aromatic imides and fatty amines. The properties and formation process of this complex were systematically studied through steady-state and time-resolved emission spectroscopy, absorption spectroscopy, mass spectrometry, paramagnetic resonance spectroscopy, and other methods. It was demonstrated that this complex can be used in fields such as photo induced polymerization, carbon dioxide photoreduction, and ultraviolet energy storage. The achievement was published in Chem under the title "Trapping Highly Reactive Photo Induced Charge Transfer Complex Between Amine and Imide by Light".
The charge transfer between molecules refers to the movement of electrons from donor molecules to acceptor molecules, and is the most important physical process in material interactions and chemical reactions. It is ubiquitous in nature and plays an indispensable role in processes such as photosynthesis and respiration; Charge transfer also has a wide range of applications in organic synthesis, energy conversion, and other fields. Therefore, unlocking new charge transfer mechanisms is crucial for understanding the complex photochemical and photophysical processes in nature, developing efficient organic synthesis methodologies, and energy conversion technologies.
If there is a strong interaction between two organic molecules through charge transfer, this pair of molecules is usually referred to as a charge transfer complex. Organic charge transfer complexes can generally be divided into two categories. One is the interaction between electron donors and acceptors in the ground state, forming the ground state electron donor acceptor complex (EDA complex); Another type is when one of the electron donors or acceptors is in an excited state and interacts with the other in the ground state to form an exciplex, which is commonly found in fields such as light-emitting diodes, semiconductors, and photovoltaic devices. Radical complexes are highly unstable and typically dissociate into electron donor and acceptor molecules in the ground state within microseconds as the complex decays from the excited state to the ground state.
In theory, by reasonably regulating the energy levels of organic electron donor and acceptor molecules and constructing a higher binding energy barrier, it is possible to achieve no interaction between the two in the ground state, while in the excited state of electrons, the donor acceptor pairs can interact through charge transfer and remain stable even in the ground state after the excited state of electrons fades away. However, so far, ground state organic electron complexes that can only be formed through excited states have not been realized. Based on previous research on the photophysical properties of imides, Professor Zhang Guoqing's research group found that when imides are used as electron acceptors and tertiary amines as electron donors, there is no significant ground state interaction between the two in solution at room temperature, but significant interaction occurs under light conditions. The spectral characteristics are similar to those of exciton complexes, and the ground state of this newly emerged complex can remain stable for a long time in an inert atmosphere (Figure 1).
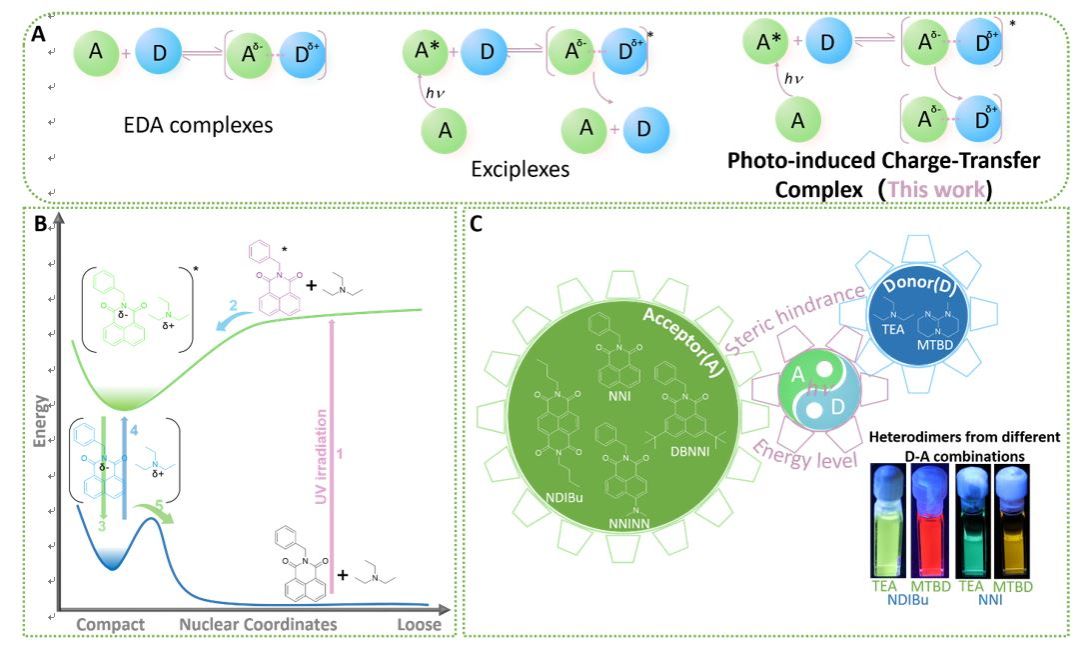
Recently, Professor Zhang Guoqing's team from the National Research Center for Microscale Matter Science at the University of Science and Technology of China in Hefei reported the discovery of a new mode of interaction between organic molecules: a stable photo induced charge transfer complex can be formed between aromatic imides and fatty amines. The properties and formation process of this complex were systematically studied through steady-state and time-resolved emission spectroscopy, absorption spectroscopy, mass spectrometry, paramagnetic resonance spectroscopy, and other methods. It was demonstrated that this complex can be used in fields such as photo induced polymerization, carbon dioxide photoreduction, and ultraviolet energy storage. The achievement was published in Chem under the title "Trapping Highly Reactive Photo Induced Charge Transfer Complex Between Amine and Imide by Light".
1. Formation and regulation of photo induced charge transfer complexes (PCTC)
Professor Zhang Guoqing's research group first selected naphthalimide and triethylamine as model compounds, and determined the existence of photo induced charge transfer complexes (PCTC) by measuring the spectral properties of the mixed system of naphthalimide and triethylamine before and after light irradiation (Figure 2). The formation mechanism of PCTC was studied by high-resolution mass spectrometry, time-resolved spectroscopy, changing the substituents of naphthalimide molecules, replacing electron donors, and other methods, proving that it indeed requires charge transfer in the excited state and subsequent de excitation of the electron excited state to form.
2. Spectral characteristics of the mixed system of naphthalimide and triethylamine before and after illumination
The research team successfully applied this system to the photo induced polymerization of acrylic monomers, photo reduction of carbon dioxide, and light energy storage and release. By releasing the stored light energy under dark conditions, the process that originally required light can also be carried out under dark conditions (Figure 3). According to the author's speculation, the formation of stable ground state complexes in solution through electronic excited states should not be limited to the intermolecular interaction mode between imide and amine molecules. It is likely to be a common but overlooked interaction, which is expected to be discovered in more molecular structures and used for new photochemical reactions.
3. Application of Imide and Amine Light Induced Charge Transfer Complex
Professor Zhang Guoqing and Associate Professor Chen Biao from the Hefei National Research Center for Microscale Material Science at the University of Science and Technology of China are the corresponding authors of this paper; Postdoctoral researcher Huang Wenhuan is the first author of this paper. This work has received funding from the Quantum Technology Innovation Program of the University of Science and Technology of China, the Important Direction Project Cultivation Fund of the University of Science and Technology of China, and the National Natural Science Foundation of China.
Paper link: https://www.sciencedirect.com/science/article/pii/S2451929424002262
(Hefei National Research Center for Microscale Material Science, Hefei National Laboratory, Ministry of Science and Technology)
|
|
https://news.sciencenet.cn/htmlnews/2024/6/524511.shtm
|






